Yesterday the world was celebrating International Women’s Day. I had planned to talk about reproductive health but got a little too carried away with this post (it’s very long, as you can see), so it didn’t go live in time. Better late than never, I guess!
If you follow me on Twitter, you might already know about my struggles with infertility. What you might not know is that, despite having symptoms since my teenage years, I was only diagnosed with polycystic ovaries in my thirties.
But let’s start from the beginning…
Research gap
For decades, women’s reproductive health was (and still is) under-researched when compared to male health. Behind this might be the fact that women of “childbearing potential” were excluded from clinical trials by the Food and Drug Administration (FDA), from 1977 to 1993 1,2. This is not even about reproductive health specifically, but any clinical trials for any drugs or conditions. For decades, almost every health decision was based on the clinical trial results for cisgender males, and any differences between male and female bodies, including important hormonal differences, were disregarded. Even female mice were excluded from most studies, as it was believed that hormonal fluctuations would only make data much noisier and harder to interpret 3. Imagine excluding half of the population from any medical research.
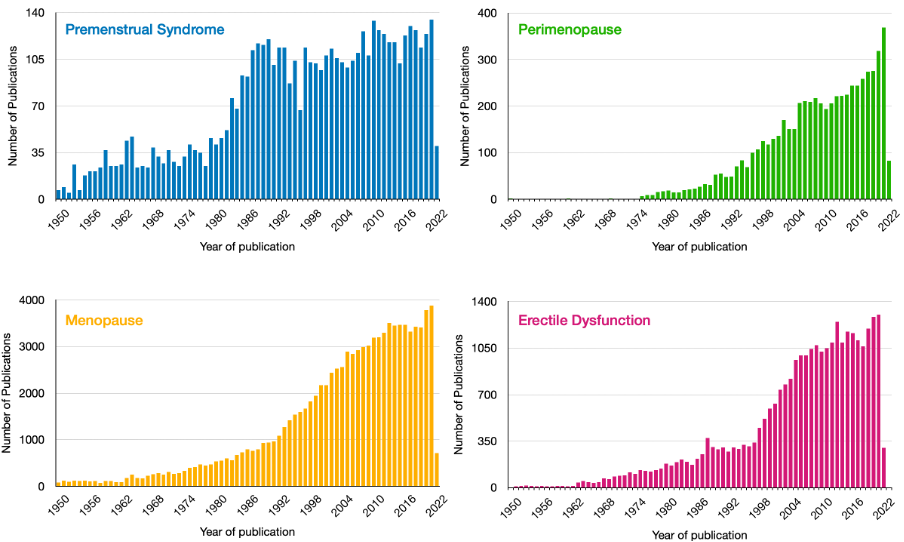
This led to a massive gap in research: while erectile disfunction affects 19% of men and premenstrual syndrome (PMS) affects 90% of women, there is 5 times more research on erectile dysfunction than PMS. This includes research on mental health issues associated with these conditions and others, such as postnatal depression or perimenopause-associated depression. The figure below shows the differences between the (unfiltered) number of scientific publications that mention PMS, perimenopause, menopause, or erectile dysfunction (data from PubMed). Granted, there are more publications on menopause than erectile dysfunction. However, the difference is still striking when we consider that menopause affects half of the world’s population.
The gap in research (and the reasons behind it) is even more frustrating when we look at overall health and find that 78% of people with autoimmune diseases are women 4, or women have shown to be underdiagnosed and misdiagnosed with neurodevelopmental disorders, such as ADHD and autism 5,6.
If you want to know more about reproductive health in general, I recommend the seminar below with experts from Cardiff University on a variety of topics: From menstruation to menopause: a reproductive mental health journey.
Medical bias
This article by The Guardian (link here) has some good insights into how medicine has historically treated women. It is important to point out that medicine is not only extremely cis-male centred but also white centred. That is a discussion for another day.
As the article points out, a fairly recent publication looked into how medical practitioners talk to, evaluate and refer to patients with endometriosis 7. It seems quite hard to believe, but some of the interviewed practitioners in this 2018 study said they often disregard the patient’s knowledge of their own body or are “suspicious” of women’s accounts of their symptoms. This was true for both male and female clinicians. However, while both tended to use the word “girls” when referring to women, female clinicians would self-correct, while male clinicians did not. Using the word “girl” to refer to adult women has been heavily discussed in the media 8. An author even “called it out” in communication back in 1983, on the use of “girls” when referring to adult patients or in a medical context with colleagues 9. However, in 2022, it is still commonly used. Referring to adult women as girls or saying that patients have “girls’ problems” is very diminishing but, sadly, commonplace.
Photo by Nadezhda Moryak from Pexels
“Do mad people get endo or does endo make you mad? It’s probably a bit of both.”, one GP in the study has said.
Women with endometriosis are still often seen as “difficult” or “hysterical” patients. It is no wonder that the median time to endometriosis diagnosis from symptom onset is 7 years 10. While endometriosis is more common in the lower abdomen or pelvis, in tissues surrounding the uterus, it can appear anywhere and affects approx. 10% of women 11. This figure is probably an underestimation, as the diagnosis requires invasive procedures.
The birth control pill as the answer to all problems
If you want to learn more about endometriosis and read personal stories, visit endometriosis-uk.org. It includes several personal stories of women with endometriosis, including Claire’s, who waited 14 years for a diagnosis of stage 4 endometriosis. Like many other women, myself included, Claire’s pain around ovulation and menstruation was put down as “a normal part of being a woman”. Claire was prescribed the combined birth control pill at the age of 13, just one year after she first got her first period. While society discusses the ethics behind administering a simple vaccine to children or using possibly life-saving puberty blockers and other hormonal treatments for teen trans people, nobody bats an eyelid when a 13-year-old is prescribed a birth control pill. In fact, it is common for pre-teens to be prescribed the combined birth control pill, and told to take it for the foreseeable future to help with “abnormal menstrual pain”, acne or irregular periods. This is despite all the listed side effects, such as deep vein thrombosis, depression, stroke, heart disease, and even increased risk of cervical and breast cancer 12, 13, 14. On the other hand, oral contraception can also have protective effects against ovarian cancer 15.
The complex nature of the benefits and drawbacks of hormonal contraception means that risks and benefits must be carefully weighed for individual patients. The question is, are they? How confident are we that healthcare professionals do a thorough analysis of risks and benefits and medical history of individual patients when they have 10-15 minutes to assess a 13-year-old with irregular periods?
The answer is complex and will depend on many factors, including the historical context (as discussed above) of reproductive health, the knowledge and experience a practitioner has on the topic (including symptoms, treatment options, tests, and diagnosis), a patient’s medical history and presentation, psychosocial factors, and the time available for consultation. These are only a few of the factors that may influence a practitioner’s decision on whether to prescribe oral contraception.
Similarly to Claire, I started menstruating at the age of twelve. Periods were extremely irregular, long, high flow and very painful. I would often get my period overnight and faint as soon as I would try getting up from bed. They were so painful that I remember sitting in exams and cramps were so bad that I used to pinch myself constantly. The pain from pinching my own skin would very temporarily provide some pain relief. I kept being reassured by doctors and every woman I knew that this was all normal and I shouldn’t be such a wimp. “It can’t possibly hurt that much”, my mom would tell me. I tried many types of pain relief and there were times when none would provide relief. PMS wasn’t easy and I would burst into tears for no apparent reason. Periods were so irregular that I would often not menstruate for two months. Doctors kept reassuring me, well into my 20s, that it was all normal, I was too young, and if I would start birth control “it would all sort itself out”.
Indeed, taking the birth control pill sorted things out for me. Until I stopped it because we wanted to start a family. For years I had minimal cramps, regular flow and no PMS. Everything came back one month after I stopped taking a combined birth control pill.
Polycystic ovaries and infertility
A few months later, I went to my GP, concerned that my menstrual cycles were too long (between 45-50 days). Again, I was reassured that there was no cause for concern and was told “as long as you have a period, it’s all good. You might just have fewer chances of getting pregnant any given month”. The NHS website provides some information on the menstrual cycle, indicating that the average period is 28 days, with anything between 21 to 40 days considered normal 16. However, a large study found that only 13% of participants had a 28-day menstrual cycle 17. It also showed that, even in a 28-day cycle, ovulation occurs after day 15, and not on day 14 as it is usually reported, even in medical textbooks. For an egg to be released from a follicle, one hormone called luteinising hormone (commonly known as LH) needs to increase enough (peak) to trigger this release. Ovulation then occurs approximately 28-36 h later. Many textbooks still show ovulation as an event that happens at the exact same time as the LH peak.
When the LH surge fails to trigger the release of the egg, the follicle eventually turns into a cyst. After this, LH may peak again, stay elevated for a period of time, or decrease. The presence of ovarian cysts indicates either polycystic ovaries (PCO) or polycystic ovarian syndrome (PCOS). PCO affects 20-25% of all women and is characterised by the presence of multiple follicles in one ovary, leading to enlarged ovaries. On the other hand, PCOS affects approx. 10% of women (prevalence can vary with ethnicity), is a metabolic disease and one of the most common causes for infertility.
If you are interested in knowing more about PCOS, I recommend the video below on the health implications and health risks of PCOS, including an increased risk of heart disease, diabetes type 2 and having children with ADHD.
Imagine my surprise when a couple of blood tests and an ultrasound scan led to me being diagnosed with PCOS at the age of 32. Turns out that the premises of “as long as you have a period it’s ok” or “you are still young and this is all normal”, were wrong. They were, in fact, signs of PCOS that were missed. Again, just like discussed above, they may have been missed for a variety of reasons. One of them was that I did not present with one of the common PCOS symptoms: weight gain 18. In fact, I was underweight until my mid-twenties and have never been overweight.
While it is possible for women with PCOS to get pregnant, PCOS is one of the main causes of infertility. However, it is treatable and it is possible for women to receive treatment that helps induce ovulation or regulate the hormonal imbalances, and conceive naturally.
It turns out that PCOS wasn’t our only cause for infertility. However, being diagnosed so late and 2 years after we started trying to conceive, does leave a bittersweet taste and a lot of questions:
- What if I had been diagnosed earlier?
- Could I have been treated before?
- Had I known I had PCOS in my teens, could I have had treatment then and not have to suffer as much?
- Did I waste time trying to get pregnant when I couldn’t?
All these questions have a great impact on women’s mental health, as we often blame ourselves for our inability to have children 19. This is even the case when male infertility is one of the factors for a couple.
So, let’s think about this for a second: women were kept out of any medical trial for decades, the balance of research being done on female/male reproductive health is skewed and reproductive health education is poor. Women are underdiagnosed or take many years to get a diagnosis for common conditions and, still, blame themselves for failing to get pregnant. It is baffling, is it not?
This happens because, while overall knowledge in reproductive health has a gap regarding women, fertility research and anything that is infertility oriented, is focused on women. This sounds really odd when we think about the fact that male infertility accounts for approx. 1/3 of infertility cases in couples. The remaining two-thirds are divided between female factors or both, with unexplained infertility accounting for only 15%.
Men are poorly supported when it comes to reproductive health
Despite being fairly common, male infertility is even more of a taboo subject than erectile dysfunction 20. In fact, a survey by Fertility Network UK has shown that men diagnosed with infertility feel emasculated and suffer from poor mental health 21. However, there is little support and treatments are so focused on the partner who will carry the pregnancy, that men (and other partners) often feel left out of the treatment process. There is also a common misconception, perhaps fuelled by all the stories in the media of famous old men having babies with their younger wives (Julio Iglesias and Michael Douglas are good examples).
However, it isn’t true that older men are more fertile than older women. In fact, fertility in men declines with age, contrary to popular belief 22. The difference here is that what we see in the media, are older men having children with younger women. If those same men tried to conceive with someone older or of similar age to them, it would be likely that conception would take longer, and the risk of malformations and miscarriage increased. That said, nobody tells men that they are “running out of time” or they are approaching their “expiry date” as they do to women.
The process of fertility treatments itself is extremely focused on women. This might be because there is only so much that can be done to treat male infertility: lifestyle changes (that may or may not help), or sperm donors. If the sperm parameters are below what is considered ideal (especially in terms of movement – motility), intracytoplasmic sperm injection (ICSI) can be used. This is when spermatozoid is injected directly into the egg, to avoid failed fertilisation due to the sperm not being able to reach the egg when both are mixed. On the other hand, despite being similar in terms of statistics, female infertility factors are varied and can be anything from birth defects to lack of progesterone. So, there is also a variety of treatments that are possible for female infertility (like surgery or medication) that are simply not possible for men.
Due to this, the process of fertility treatments is very focused on the partner who can get pregnant. It is that partner who goes through multiple internal vaginal scans a week, many blood tests to check for hormone levels, multiple daily injections and other medication, with their consequent side effects, and even a surgical procedure under sedation to remove the eggs from the ovaries. Most of the physical toll of fertility treatments is on the woman. Also, a lot of the mental load, as it will be mostly the woman organising and planning treatments, and liaising with clinics, as they will be the ones going to the medical appointments.
There is only one thing required of men undergoing fertility treatment: to provide the sperm sample. And that is if the couple is not using a sperm donor. This means that men are excluded or set aside from most of this process and have reported feeling very unsupported throughout. This was only made worse with the COVID-19 pandemic, when fertility clinics worldwide closed, processes became slower, and partners stopped being allowed for any procedure. In fact, in our former NHS fertility clinic, partners still are not allowed in for any part of the process, other than tests and treatment plans. This also includes not being allowed in for when the embryo is being transferred or supporting the partner when they are recovering from the surgical egg collection. Two years after the start of the pandemic, I had to go through a complicated embryo transfer alone and take a photograph of a screen, while my husband waited in the car park.
In the years of infertility, not one healthcare professional asked how my husband feels, despite asking me many times throughout. Not even the counsellor during counselling sessions asked how he was feeling. The whole process is focused on how I feel and what I go through, with little thought about how the partner might feel.
Even after pregnancy happens, there is little thought of the partner, whether things go wrong or not. Miscarriage is very common (1 in 4 of all pregnancies) and causes are mostly unknown, especially for early miscarriages in the first trimester. Women seek reassurance from medical professionals that it will not happen again, but practitioners do not know more than simple statistics on whether it is likely to happen or not. It is still commonplace around the world, especially in countries with public health care, that nothing will be done or investigated until the woman suffers 3 miscarriages. Of course, miscarriage affects the pregnant partner more, physically. But this does not mean that healthcare teams, and society in general, should relegate the role of the partner solely to that of support. Men will grieve pregnancy loss and suffer the loss itself, and see their partner go through the emotional and physical burden of pregnancy loss. A colleague has recently shared his very powerful account of pregnancy loss and infertility through the eyes of the male partner. You can read it here.
Reproductive Health education needs improvement
Reproductive health education has a long way to go. It is important that whoever wants and feels they can, speaks openly about reproductive health, infertility, and loss. It helps others feels less alone, more supported, and possibly more informed. I personally believe that there is a massive focus on preventing teen pregnancy and sexually-transmitted diseases, which is of great importance. But this does not match the level of reproductive health education we need to transmit to the younger generations. The focus is so high on getting teens to fear getting pregnant, that it prevents them from actually understanding how their body works and how to look for signs that anything might be wrong.
We need to empower the younger generations to take care and understand their bodies and have ownership of their reproductive health.
Andreia
1 – https://www.fda.gov/science-research/womens-health-research/regulations-guidance-and-reports-related-womens-health
2 – https://www.theguardian.com/lifeandstyle/2019/nov/13/the-female-problem-male-bias-in-medical-trials
3 – https://www.semanticscholar.org/paper/Biomedical-research.-Of-mice-and-women%3A-the-bias-in-Wald-Wu/1654940bba9878fd7f732440aeb72b54d18790a6?p2df
4 – https://pubmed.ncbi.nlm.nih.gov/15550215/
5 – https://www.adhdcentre.co.uk/why-is-adhd-underdiagnosed-in-women/
6 – https://theconversation.com/autism-is-still-underdiagnosed-in-girls-and-women-that-can-compound-the-challenges-they-face-176036#:~:text=Girls%20who%20don’t%20get,or%20before%2C%20an%20autism%20diagnosis.
7 – https://journals.sagepub.com/doi/10.1177/0959353518815704
8 – https://www.forbes.com/sites/forbescoachescouncil/2021/08/09/why-calling-women-girls-is-a-bigger-deal-than-you-may-think/?sh=50a92c932fda
9 – https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1875836/
10 – https://academic.oup.com/humrep/article/18/4/756/596537
11 – https://www.uclahealth.org/obgyn/endometriosis
12 – https://pubmed.ncbi.nlm.nih.gov/11943255/
13 – https://pubmed.ncbi.nlm.nih.gov/34962522/
14 – https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6322132/
15 – https://pubmed.ncbi.nlm.nih.gov/24090961/
16 – https://www.nhs.uk/conditions/periods/fertility-in-the-menstrual-cycle/
17 – https://www.nature.com/articles/s41746-019-0152-7
18 – https://www.nhs.uk/conditions/polycystic-ovary-syndrome-pcos/
19 – https://ir.lib.uwo.ca/cgi/viewcontent.cgi?article=1337&context=philosophypub
20 – https://www.insider.com/when-facing-infertility-women-are-more-likely-to-blame-themselves-2020-6
21 – https://fertilitynetworkuk.org/male-infertility-is-emasculating-its-treatment-one-sided-and-insensitive-and-emotional-support-is-lacking-first-qualitative-survey-of-mens-experiences-of-fertility-problems-finds/
22 – https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3253726/